 产品供求
产品供求
头孢孟多酯钠
- 基本信息
- 制备方法及用途
- 物化性质
- 安全信息
- 毒理性
头孢孟多酯钠 基本信息
- 中文名称:
- 头孢孟多酯钠
- 中文别名:
- 头孢孟多钠;
(6R,7R)-3-[[(1-甲基-1H-四唑-5-基)硫]甲基]-7-[(R)-2-羟基-2-苯乙酰氨基]-8-氧代-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-甲酸钠;
头孢孟多酸钠
- 英文名称:
- Cefamandole sodium
- 英文别名:
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2R)-2-hydroxy-2-phenylacetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, sodium salt (1:1), (6R,7R)-;
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[(hydroxyphenylacetyl)amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, monosodium salt, [6R-[6α,7β(R*)]]-;
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2R)-hydroxyphenylacetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, monosodium salt, (6R,7R)- (9CI);
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-mandelamido-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, monosodium salt, D- (8CI);
Cefamandole sodium;
Cefamandole sodium salt;
Kefdole;
Sodium 7-D-mandelamido-3-(1-methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-carboxylate;
Sodium [6R-[6α,7β(R*)]]-7-[(hydroxyphenylacetyl)amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;
Sodium cefamandole
- CAS No.:
- 30034-03-8
- 分 子 式:
-
C18H18N6O5S2.Na
- 分 子 量:
- 484.49
- 精确分子量:
- 485.06778
- PSA:
- 203.97000
- EINECS:
- 250-009-0
- InChI:
- InChI=1/C18H18N6O5S2.Na/c1-23-18(20-21-22-23)31-8-10-7-30-16-11(15(27)24(16)12(10)17(28)29)19-14(26)13(25)9-5-3-2-4-6-9;/h2-6,11,13,16,25H,7-8H2,1H3,(H,19,26)(H,28,29);/q;+1/p-1/t11-,13-,16-;/m1./s1
- 危险品标志:
-
 Xn
Xn
- 风险术语:
-
R36/37/38;R42/43
- 安全术语:
-
S26;S36
- 分子结构式:
-
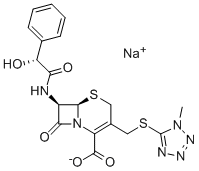
- SDS:
- 查看
头孢孟多酯钠 物化性质
- 外观与性状:
- 白色固体
- 熔点:
- >175ºC (dec.)
- 存储条件/存储方法:
- 库房低温通风干燥
头孢孟多酯钠 安全信息
- WGK_Germany:
- 2
德国有关水污染物质的分类清单
- 危险类别码:
- R36/37/38;R42/43
- 安全说明:
- 26-36
- RTECS号:
- XI0389000
- 危险标志:
- Xn: Harmful;
头孢孟多酯钠 毒理性
CHEMICAL IDENTIFICATION
- RTECS NUMBER :
- XI0389000
- CHEMICAL NAME :
- 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic
acid, 7-mandelamido-3-(((1-methyl-
1H-tetrazol-5-yl)thio)methyl)-8-oxo-, monosodium
salt, D-
- CAS REGISTRY NUMBER :
- 30034-03-8
- LAST UPDATED :
- 199507
- DATA ITEMS CITED :
- 18
- MOLECULAR FORMULA :
- C18-H17-N6-O5-S2.Na
- MOLECULAR WEIGHT :
- 484.52
- WISWESSER LINE NOTATION :
- T46 ANV ES GUTJ CMVYQR& HVO G1S- ET5NNNNJ A1 &-NA-
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- >16600 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka
Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959-
Volume(issue)/page/year: 26,115,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 12100 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka
Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959-
Volume(issue)/page/year: 26,115,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 4410 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka
Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959-
Volume(issue)/page/year: 26,115,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- >19900 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka
Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959-
Volume(issue)/page/year: 26,115,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 10300 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka
Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959-
Volume(issue)/page/year: 26,115,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 4460 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka
Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959-
Volume(issue)/page/year: 26,115,1984
- TYPE OF TEST :
- LD - Lethal dose
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Rodent - rabbit
- DOSE/DURATION :
- >2 gm/kg
- TOXIC EFFECTS :
- Behavioral - food intake (animal)
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure,
acute tubular necrosis)
Kidney, Ureter, Bladder - other changes in urine composition
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),701,1979
** OTHER MULTIPLE DOSE TOXICITY DATA **
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Rodent - rabbit
- DOSE/DURATION :
- 2800 mg/kg/7D-I
- TOXIC EFFECTS :
- Related to Chronic Data - death
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),701,1979
** REPRODUCTIVE DATA **
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 11 gm/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - musculoskeletal system
Reproductive - Specific Developmental Abnormalities - urogenital system
Reproductive - Effects on Newborn - live birth index (measured after birth)
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 11 gm/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - delayed effects
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 5500 mg/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 13500 mg/kg
- SEX/DURATION :
- female 17-22 day(s) after conception
lactating female 21 day(s) post-birth
- TOXIC EFFECTS :
- Reproductive - Maternal Effects - ovaries, fallopian tubes
Reproductive - Maternal Effects - uterus, cervix, vagina
Reproductive - Effects on Newborn - physical
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),682,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- DOSE :
- 5500 mg/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - musculoskeletal system
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
Reproductive - Effects on Newborn - delayed effects
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- DOSE :
- 22 gm/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - urogenital system
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- DOSE :
- 27 gm/kg
- SEX/DURATION :
- female 17-22 day(s) after conception
lactating female 21 day(s) post-birth
- TOXIC EFFECTS :
- Reproductive - Maternal Effects - ovaries, fallopian tubes
Reproductive - Maternal Effects - parturition
Reproductive - Effects on Newborn - physical
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),682,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 780 mg/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured
before birth)
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 195 mg/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- DOSE :
- 390 mg/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured
before birth)
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
- REFERENCE :
- NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki,
Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year:
27(Suppl 5),658,1979
湖北云镁科技有限公司
- 产品介绍:
-
产品名称:头孢孟多钠
Cas号:30034-03-8
纯度:99%
包装信息:359kg,1kg铝箔袋/氟化瓶,10kg铝听/纸箱,25kg纸板桶/编织袋/塑料桶
价格:1.0元 - 359.0元
- 联系方式:
-
联系人:方经理
电话:027-59206672
手机:18327059871
上海吉至生化科技有限公司
- 产品介绍:
-
产品名称:头孢孟多酯钠
Cas号:30034-03-8
纯度:95%
包装信息:10mgea
价格:548.0元
- 联系方式:
-
联系人:江珊
电话:021-57520831
手机:15316869680
山东默派生物科技有限公司
- 产品介绍:
-
产品名称:头孢孟多钠
Cas号:30034-03-8
纯度:99%
包装信息:100kg
价格:100.0元
- 联系方式:
-
联系人:张经理
电话:13070690626
手机:13070690626
湖北江民泰华化工有限公司
- 产品介绍:
-
产品名称:头孢孟多酯钠
Cas号:30034-03-8
纯度:99%
包装信息:200kg,25kg
价格:洽谈
- 联系方式:
-
联系人:张经理
电话:13396038321
手机:13396038321
湖北恩星生物科技有限公司
- 产品介绍:
-
产品名称:头孢孟多钠
Cas号:30034-03-8
纯度:
包装信息:500kg,400kg,300kg,200kg,100kg
价格:64.0元 - 100.0元
- 联系方式:
-
联系人:邵经理
电话:16621771607
手机:16621771607
湖北魏氏化学试剂股份有限公司
- 产品介绍:
-
产品名称:头孢孟多酯钠
Cas号:30034-03-8
纯度:
包装信息:洽谈
价格:洽谈
- 联系方式:
-
联系人:刘涛
电话:027-59101766
手机:13125137661
湖北实兴化工有限公司
- 产品介绍:
-
产品名称:头孢孟多钠
Cas号:30034-03-8
纯度:99%
包装信息:500kg,200kg,50kg,25kg,1kg
价格:1.0元 - 10.0元
- 联系方式:
-
联系人:张妍
电话:13554155445
手机:13554155445
山东昌儒生物科技有限公司
- 产品介绍:
-
产品名称:头孢孟多钠
Cas号:30034-03-8
纯度:99以上
包装信息:25公斤,1公斤
价格:洽谈
- 联系方式:
-
联系人:熊
电话:15342250425
手机:15342250425
苏州市晶华化工有限公司
- 产品介绍:
-
产品名称:头孢孟多钠
Cas号:30034-03-8
纯度:98%-99%
包装信息:2kg
价格:洽谈
- 联系方式:
-
联系人:屠先生
电话:0512-52511818
手机:13906222958
湖北盛天恒创生物科技有限公司
- 产品介绍:
-
产品名称:头孢孟多酯钠
Cas号:30034-03-8
纯度:
包装信息:洽谈
价格:洽谈
- 联系方式:
-
联系人:陈经理
电话:027-87676898 18971248750 18971247857
手机:18971248750
 Xn
Xn