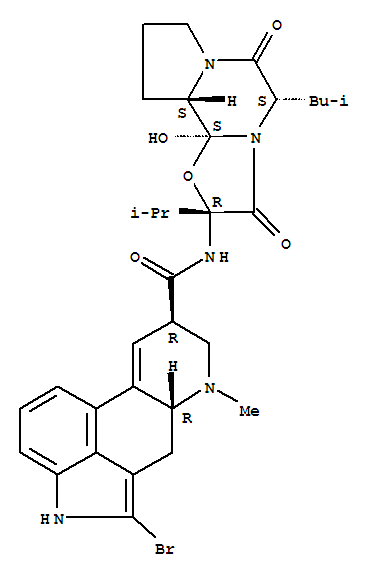
由麦角隐亭和N-溴丁二酸亚胺反应得到。
本品具有多巴胺受体的兴奋作用。可透过血脑屏障,进入中枢神经内,激动多巴胺受体和抑制催乳素及生长激素分泌作用。临床用于帕金森氏症、肢端肥大症和乳溢症等。
1性状:白色结晶性粉末。
2熔点:215-218℃(分解)。
1、 摩尔折射率:165.35
2、 摩尔体积(cm3/mol):429.3
3、 等张比容(90.2K):1257.7
4、 表面张力(dyne/cm):73.6
5、 极化率(10-24cm3):65.55
1.疏水参数计算参考值(XlogP):3.8
2.氢键供体数量:3
3.氢键受体数量:6
4.可旋转化学键数量:5
5.互变异构体数量:8
6.拓扑分子极性表面积118
7.重原子数量:43
8.表面电荷:0
9.复杂度:1230
10.同位素原子数量:0
11.确定原子立构中心数量:6
12.不确定原子立构中心数量:0
13.确定化学键立构中心数量:0
14.不确定化学键立构中心数量:0
15.共价键单元数量:1