C19H16ClFN2O
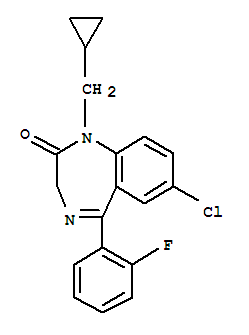
化合物(Ⅰ)在氯化氢-乙醇中,于70℃加热,环合得到化合物(1I)。然后氨解为酰胺(Ⅲ),用氢化铝锂还原为胺(Ⅳ)。最后用三氧化铬,在乙酸-水中,于室温氧化扩环,得到氟托西泮。
苯并二氮杂卓类药物。作用机制和其它苯并二氮杂卓类药物相似,但较持久,每日只需服用一次。用于神经症状如不安、紧张、抑郁、易疲劳及睡眠障碍,还用于心身疾病如高血压、慢性胃炎、过敏性大肠为症候群产生的不安、紧张、抑郁、易疲劳及睡眠障碍。
1.性状:结晶。
2.熔点:118~122℃。
急性毒性LD50雄、雌小鼠,雄、雌大鼠(mg/kg):2640,2430,13760,10060口服;2400,2110,2460,2230腹腔注射;全部>5000皮下注射。
1、 摩尔折射率:92.37
2、 摩尔体积(cm3/mol):246.4
3、 等张比容(90.2K):657.1
4、 表面张力(dyne/cm):50.5
5、 极化率(10-24cm3):36.62
1.疏水参数计算参考值(XlogP):无
2.氢键供体数量:0
3.氢键受体数量:3
4.可旋转化学键数量:3
5.互变异构体数量:2
6.拓扑分子极性表面积32.7
7.重原子数量:24
8.表面电荷:0
9.复杂度:522
10.同位素原子数量:0
11.确定原子立构中心数量:0
12.不确定原子立构中心数量:0
13.确定化学键立构中心数量:0
14.不确定化学键立构中心数量:0
15.共价键单元数量:1