Section 1. Chemical Product and Company Identification
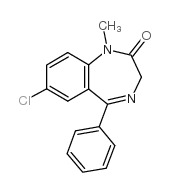
Diazepam
Common Name/
Trade Name
Diazepam
Section 4. First Aid Measures
Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least
Eye Contact
15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention.
In case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Remove
Skin Contact
contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical
attention.
Serious Skin Contact Wash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek immediate
medical attention.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get
medical attention.
Serious Inhalation Not available.
Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an
Ingestion
unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight
clothing such as a collar, tie, belt or waistband.
Serious Ingestion Not available.
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
Not available.
Flash Points
Not available.
Flammable Limits
These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...), halogenated compounds.
Products of Combustion
Fire Hazards in Presence of Slightly flammable to flammable in presence of heat.
Non-flammable in presence of shocks.
Various Substances
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards in
Risks of explosion of the product in presence of static discharge: Not available.
Presence of Various
Substances
SMALL FIRE: Use DRY chemical powder.
Fire Fighting Media
LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
and Instructions
Not available.
Special Remarks on
Fire Hazards
Not available.
Special Remarks on
Explosion Hazards
Section 6. Accidental Release Measures
Use appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading
Small Spill
water on the contaminated surface and dispose of according to local and regional authority requirements.
Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on
Large Spill
the contaminated surface and allow to evacuate through the sanitary system.
Diazepam
Section 7. Handling and Storage
Keep locked up.. Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk,
Precautions
evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not
breathe dust. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory
equipment. If ingested, seek medical advice immediately and show the container or the label. Avoid contact with
skin and eyes. Keep away from incompatibles such as oxidizing agents, alkalis.
Light Sensitive. Store in light-resistant containers. Keep container tightly closed. Keep container in a cool,
Storage
well-ventilated area. Do not store above 25°C (77° F).
Section 8. Exposure Controls/Personal Protection
Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
Engineering Controls
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Splash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves.
Personal Protection
Personal Protection in Case Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used to
avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE
of a Large Spill
handling this product.
Not available.
Exposure Limits
Section 9. Physical and Chemical Properties
Solid. (crystalline powder.) Odorless.
Physical state and O dor
appearance
Not available.
Taste
284.7 g/mole
Molecular Weight
Off-white. Yellow.
Color
Not available.
pH (1% soln/water)
Not available.
Boiling Point
132°C (269.6°F)
Melting Point
Not available.
Critical Temperature
Not available.
Specific Gravity
Not applicable.
Vapor Pressure
Not available.
Vapor Density
Not available.
Volatility
Not available.
Odor Threshold
The product is more soluble in oil; log(oil/water) = 3
Water/Oil Dist. Coeff.
Not available.
Ionicity (in Water)
Not available.
Dispersion Properties
Very slightly soluble in cold water.
Solubility
Insoluble in diethyl ether.
Solubility in Chloroform is 1 g/ 2 ml chloroform.
Sparingly soluble in propylene glycol.
Solubility in ether is 1mg/39 ml ether.
Solubility in water is 3 mg/1 ml water at 25 C.
Solubility in alcohol is 62.5 mg/ml alcohol at 25 C
Diazepam
Section 10. Stability and Reactivity Data
The product is stable.
Stability
Not available.
Instability Temperature
Conditions of Instability Excess heat
Incompatibility with various Reactive with oxidizing agents, alkalis.
substances
Not available.
Corrosivity
Sensitive to light.
Special Remarks on
Incompatible with mineral acid.
Reactivity
Not available.
Special Remarks on
Corrosivity
Will not occur.
Polymerization
Section 11. Toxicological Information
Inhalation. Ingestion.
Routes of Entry
Acute oral toxicity (LD50): 48 mg/kg [Mouse].
Toxicity to Animals
Acute dermal toxicity (LD50): 800 mg/kg [Mouse].
CARCINOGENIC EFFECTS: 3 (Not classifiable for human.) by IARC.
Chronic Effects on Humans
MUTAGENIC EFFECTS: Mutagenic for bacteria and/or yeast.
TERATOGENIC EFFECTS: Classified POSSIBLE for human.
May cause damage to the following organs: central nervous system (CNS).
Hazardous in case of ingestion.
Other Toxic Effects on
Slightly hazardous in case of skin contact (irritant), of inhalation.
Humans
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on It crosses the placenta readily. May cause adverse reproductive effects and birth defects. It has been reported to
Chronic Effects on Humans increase the risk of birth defects when used during the first trimester of pregancy. Chronic use or therapeutic use late
in pregnancy may also adversely affect the newborn.
May cause cancer based on animal data. No human data found.
Special Remarks on other Acute Potential Health Effects:
Toxic Effects on Humans Skin and Eyes: May cause skin and eye irritation.
Inhalation: May cause respiratory tract irritation.
Ingestion: Harmful if swallowed. May cause gastrointestinal tract irritation. May affect behavior/central nervous
system. Symptoms may include drowsiness, dizziness, slurred speech, muscle contraction, clumsiness, anxiety,
confusion, weakness, mental depression, muscle spasm, false sense of well-being, decreased reflexes coma, seizures,
shakiness, staggering, delirium, lethargy, hallucinations, excitement and irritability. It may also affect the
cardiovascular system (fast pounding, irregular heartbeat, hypotension, hypertension, disarthria, cardiac arrest), the
urinary system, respiratory system (respiratory depression) and may cause changes in vision (diplopia, visual field
changes).
Chronic Potential Health Effects:
Skin: Prolonged or repeated skin contact may cause allergic reaction.
Ingestion: Prolonged or repeated ingestion may affect behavior/central nervous system( with symptoms similar to
acute exposure), endrocrine system, brain, liver (fatty liver degeneration), urinary system, metabolism, blood
(normocytic anemia) and may cause allergic reaction, and psychological or physical dependence.
Inhalation: Prolonged or repeated inhalation may cause allergic reaction.
Diazepam
Section 12. Ecological Information
Not available.
Ecotoxicity
Not available.
BOD5 and COD
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may
arise.
The products of degradation are as toxic as the product itself.
Toxicity of the Products
of Biodegradation
Not available.
Special Remarks on the
Products of Biodegradation
Section 13. Disposal Considerations
Waste must be disposed of in accordance with federal, state and local environmental control
Waste Disposal
regulations.
Section 14. Transport Information
CLASS 6.1: Poisonous material.
DO T Cl assi fi cati on
UNNA: 2811 : Toxic solid, organic, n.o.s. (Diazepam) PG: III
Identification
Not available.
Special Provisions for
Transport
DO T (Pi ctograms)
Section 15. Other Regulatory Information and Pictograms
California prop. 65: This product contains the following ingredients for which the State of California has found to
Federal and State
cause cancer, birth defects or other reproductive harm, which would require a warning under the statute: Diazepam
Regulations
California prop. 65: This product contains the following ingredients for which the State of California has found to
cause birth defects which would require a warning under the statute: Diazepam
Massachusetts RTK: Diazepam
New Jersey: Diazepam
TSCA 8(b) inventory: Diazepam
California prop. 65: This product contains the following ingredients for which the State of California has found to
California
cause cancer which would require a warning under the statute: No products were found.
Proposition 65
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has found to
cause birth defects which would require a warning under the statute: Diazepam
OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200).
Other Regulations
EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No.
207-122-5).
Canada: Listed on Canadian Domestic Substance List (DSL).
China: Not listed on National Inventory.
Japan: Not listed on National Inventory (ENCS).
Korea: Listed on National Inventory (KECI).
Philippines: Not listed on National Inventory (PICCS).
Australia: Listed on AICS.
WHMIS (Canada) CLASS D-1B: Material causing immediate and serious toxic effects (TOXIC).
Other Classifications
DSCL (EEC) R22- Harmful if swallowed. S2- Keep out of the reach of children.
R63- Possible risk of harm to the unborn S46- If swallowed, seek medical advice
child. immediately and show this container or label.
Diazepam
Health Hazard
HMIS (U.S.A.) 2 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
2 0 Reactivity
Health
Reactivity 0
Specific hazard
Personal Protection
E
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG(Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
Lab coat.
Dust respirator. Be sure to use an
approved/certified respirator or equivalent.
Diazepam
CALL (310) 516-8000
Notice to Reader
All chemicals may pose unknown hazards and should be used withcaution. This Material Safety Data Sheet (MSDS) applies only to the material as packaged. If this product is combined with other materials,
deteriorates, or becomes contaminated, it may pose hazards not mentioned inthis MSDS. It shall be the user's responsibility to develop proper methods of handling and personal protection based onthe actual
SECTION 16 - ADDITIONAL INFORMATION
N/A