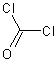
1.当需要少量光气时,可将四氯化碳与硫酸反应。将四氯化碳加热至55-60℃,滴加入发烟硫酸,即发生逸出光气,如需使用液态光气,则将发生的光气加以冷凝
工业上通常采用一氧化碳与氯气的反应得到光气。
2.工业制法以二氧化碳和氯气作原料,以活性炭催化反应,温度控制在60~150℃之间生成的气体冷却到-25℃至-30℃,液化装入钢瓶中。
实验室制法以CCl4和发烟硫酸为原料,在烧瓶中加入四氯化碳,加热至沸腾后,滴加入30%的发烟硫酸,此时生成光气COCl2,将之冷凝,即可得到液体COCl2。
市售品中含有很多杂质,如CO2、CO、空气等,含量均在1%左右,可以采用如下方法提纯[11]:
(1)将气体导入烧瓶中,用冰盐水冷却液化,然后在烧瓶上装上球形回流冷凝管通水冷凝,冷凝管和装满7~8℃的水的亨普尔气体量管连接,量管与用冰盐水冷却的冷凝器相连接,冷凝管和接收器相连接,除去烧瓶外的冷冻剂(冰盐水)后,液体即会蒸发,冷却接收器便可得到冷凝下来的COCl2。
(2)将气体导入烧瓶中,用冰盐水使之冷凝后,再撤去冰盐水,待冷凝物的约1/5蒸发后,在高真空下分馏残留物至所有的馏分都保持相同的蒸气压,纯COCl2的蒸气压为556.5mmHg。
1.用于合成氨基甲酸酯类杀虫剂,聚氨酯硬泡、软泡、弹性体、人造革的重要原料,也有的特殊品种用于粘结剂
2.制碳酸酯、氯甲酸酯、异氰酸酯、异腈、醛、氯代烷、酸酐,与腈生成杂环。
3.用于有机合成,特别是制造异氰酸酯和聚氨酯等,还用于制造染料、橡胶、农药和塑料等。[23]
1.稳定性[18] 稳定
2.禁配物[19] 水、醇类、碱类
3.避免接触的条件[20] 潮湿空气、受热
4.聚合危害[21] 不聚合
1.性状:纯品为无色有特殊气味的气体,低温时为黄绿色液体。[1]
2.熔点(ºC):-127.9~-118[2]
3.沸点(ºC):8.2[3]
4.相对密度(水=1):1.4[4]
5.相对蒸气密度(空气=1):3.4[5]
6.饱和蒸气压(kPa):161.6(20ºC)[6]
7.临界温度(ºC):182[7]
8.临界压力(MPa):5.67[8]
9.辛醇/水分配系数:-0.710[9]
10.溶解性:微溶于水,溶于芳烃、苯、四氯化碳、氯仿、乙酸等多数有机溶剂。[10]
11.液相标准热熔(J·mol-1·K-1):112.0
12.气相标准声称热(焓)( kJ·mol-1) :-219.1
13.气相标准熵(J·mol-1·K-1) :283.87
14.气相标准生成自由能( kJ·mol-1):-208.4
15.气相标准热熔(J·mol-1·K-1):57.70
1.急性毒性[11] LC50:1400mg/m3(大鼠吸入,1/2h)
2.刺激性 暂无资料
3.亚急性与慢性毒性[12] 动物吸入0.0008mg/L,每天5h,共5d,40%出现肺水肿。
4.其他[13]
LCLo:50ppm(人吸入,5min),360mg/m3(人吸入30min)
TCLo:25ppm(人吸入,30min)
1.生态毒性[14] LD50:60mg/L(24h)(鱼)
2.生物降解性[15]
好氧生物降解(h):168~672
厌氧生物降解(h):678~2688
3.非生物降解性[16]
光解最大光吸收波长范围(nm):234.2~274.5
一级水解半衰期(h):1.9
4.其他有害作用[17] 该物质对环境有危害,应特别注意对空气、水环境及水源的污染。
|
第一部分:化学品名称 |
1、摩尔折射率:16.4
2、摩尔体积(cm3/mol):64.9
3、等张比容(90.2K):153.8
4、表面张力(dyne/cm):31.5
5、介电常数:无可用
6、偶极距(10-24cm3):无可用
7、极化率:6.5
1.疏水参数计算参考值(XlogP):1.8
2.氢键供体数量:0
3.氢键受体数量:1
4.可旋转化学键数量:0
5.互变异构体数量:无
6.拓扑分子极性表面积17.1
7.重原子数量:4
8.表面电荷:0
9.复杂度:29
10.同位素原子数量:0
11.确定原子立构中心数量:0
12.不确定原子立构中心数量:0
13.确定化学键立构中心数量:0
14.不确定化学键立构中心数量:0
15.共价键单元数量:1