 产品供求
产品供求
丁酸氢化可的松
- 基本信息
- 制备方法及用途
- 物化性质
- 安全信息
- 毒理性
- 结构与计算化学
- 上游产品
- 下游产品
丁酸氢化可的松 基本信息
- 中文名称:
- 丁酸氢化可的松
- 中文别名:
- 丁酸氢化可的松;
氢化可的松17-丁酸酯;
17-丁酸皮质醇酯;
氢化可的松17丁酸杂质;
17-丁酸11β,17,21-三羟基孕-4-烯-3,20-二酮;
氢化可的松丁酸酯
- 英文名称:
- hydrocortisone 17-butyrate
- 英文别名:
- cortisol 17-butyrate;
11β,17,21-Trihydroxypregn-4-ene-3,20-dione 17-Butyrate;
Hydrocortisone 17-butyrate;
[(8S,9S,10R,11S,13S,14S,17R)-11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] butanoate;
Hydrocortisone 17-Butyrate;
Hydrocortisone butyrate;
Cortisol 17-Butyrate
- CAS No.:
- 13609-67-1
- 分 子 式:
-
C25H36O6
- 分 子 量:
- 432.55
- 精确分子量:
- 432.25100
- PSA:
- 100.90000
- EINECS:
- 237-093-4
- InChI:
- InChI=1/C25H36O6/c1-4-5-21(30)31-25(20(29)14-26)11-9-18-17-7-6-15-12-16(27)8-10-23(15,2)22(17)19(28)13-24(18,25)3/h12,17-19,22,26,28H,4-11,13-14H2,1-3H3/t17-,18?,19-,22?,23-,24-,25-/m0/s1
- 危险品标志:
-
- 风险术语:
-
- 安全术语:
- 分子结构式:
-
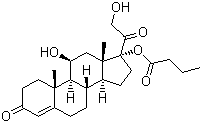
- SDS:
- 查看
丁酸氢化可的松 物化性质
- 密度:
- 1.23g/cm3
- 沸点:
- 585.6ºC at 760mmHg
- 闪点:
- 194ºC
- 折射率:
- 1.564
- 蒸汽压:
- 3.76E-16mmHg at 25°C
丁酸氢化可的松 毒理性
CHEMICAL IDENTIFICATION
- RTECS NUMBER :
- GM9002000
- CHEMICAL NAME :
- Cortisol, 17-butyrate
- CAS REGISTRY NUMBER :
- 13609-67-1
- LAST UPDATED :
- 199609
- DATA ITEMS CITED :
- 22
- MOLECULAR FORMULA :
- C25-H36-O6
- MOLECULAR WEIGHT :
- 432.61
- WISWESSER LINE NOTATION :
- L E5 B666 OV MUTJ A1 CQ E1 FV1Q FOV3
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- >3 gm/kg
- TOXIC EFFECTS :
- Nutritional and Gross Metabolic - weight loss or decreased weight gain
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intraperitoneal
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- >3 gm/kg
- TOXIC EFFECTS :
- Behavioral - changes in motor activity (specific assay)
Gastrointestinal - hypermotility, diarrhea
Kidney, Ureter, Bladder - hematuria
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- >3 gm/kg
- TOXIC EFFECTS :
- Behavioral - changes in motor activity (specific assay)
Gastrointestinal - hypermotility, diarrhea
Kidney, Ureter, Bladder - hematuria
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- >3 gm/kg
- TOXIC EFFECTS :
- Nutritional and Gross Metabolic - weight loss or decreased weight gain
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intraperitoneal
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 1550 mg/kg
- TOXIC EFFECTS :
- Behavioral - somnolence (general depressed activity)
Kidney, Ureter, Bladder - hematuria
Skin and Appendages - hair
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 2150 mg/kg
- TOXIC EFFECTS :
- Sense Organs and Special Senses (Eye) - ptosis
Behavioral - changes in motor activity (specific assay)
Lungs, Thorax, or Respiration - respiratory depression
- REFERENCE :
- JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological
Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku,
Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 6(Suppl 1),1,1981
** OTHER MULTIPLE DOSE TOXICITY DATA **
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Administration onto the skin
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 87500 mg/kg/35D-C
- TOXIC EFFECTS :
- Liver - changes in liver weight
Endocrine - changes in thymus weight
Blood - changes in erythrocyte (RBC) count
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 20,225,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Administration onto the skin
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 183 mg/kg/1Y-I
- TOXIC EFFECTS :
- Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
Blood - changes in erythrocyte (RBC) count
Blood - changes in leukocyte (WBC) count
- REFERENCE :
- KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda
Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960-
Volume(issue)/page/year: 21,1437,1987
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Administration onto the skin
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 7500 ug/kg/30D-I
- TOXIC EFFECTS :
- Endocrine - changes in thymus weight
Blood - changes in bone marrow (not otherwise specified)
Nutritional and Gross Metabolic - weight loss or decreased weight gain
- REFERENCE :
- JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological
Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku,
Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 7(Suppl
1),15,1982
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 90 mg/kg/90D-I
- TOXIC EFFECTS :
- Blood - changes in erythrocyte (RBC) count
Skin and Appendages - hair
Nutritional and Gross Metabolic - weight loss or decreased weight gain
- REFERENCE :
- KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda
Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960-
Volume(issue)/page/year: 21,1309,1987
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 15 mg/kg/30D-C
- TOXIC EFFECTS :
- Endocrine - changes in thymus weight
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
Related to Chronic Data - changes in testicular weight
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 91 mg/kg/26W-C
- TOXIC EFFECTS :
- Lungs, Thorax, or Respiration - changes in lung weight
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
Related to Chronic Data - changes in testicular weight
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,991,1974
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 15600 ug/kg/26W-I
- TOXIC EFFECTS :
- Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
Blood - changes in leukocyte (WBC) count
Blood - changes in platelet count
- REFERENCE :
- JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological
Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku,
Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 6(Suppl 1),6,1981
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Administration onto the skin
- SPECIES OBSERVED :
- Mammal - dog
- DOSE/DURATION :
- 90 mg/kg/90D-C
- TOXIC EFFECTS :
- Liver - other changes
Endocrine - adrenal cortex hypoplasia
Blood - other changes
- REFERENCE :
- KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda
Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960-
Volume(issue)/page/year: 21,1389,1987
** REPRODUCTIVE DATA **
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Administration onto the skin
- DOSE :
- 16500 ug/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
- REFERENCE :
- YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu
Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972-
Volume(issue)/page/year: 9,3045,1981
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 54 mg/kg
- SEX/DURATION :
- female 9-14 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - post-implantation mortality (e.g. dead and/or
resorbed implants per total number of implants)
Reproductive - Effects on Embryo or Fetus - fetal death
Reproductive - Effects on Newborn - live birth index (measured after birth)
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,1035,1974
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 1100 ug/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 21,441,1981
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 55 mg/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
Reproductive - Effects on Newborn - physical
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 20,67,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 6 mg/kg
- SEX/DURATION :
- female 7-12 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - musculoskeletal system
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,1035,1974
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Administration onto the skin
- DOSE :
- 1300 ug/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - pre-implantation mortality (e.g. reduction in
number of implants per female; total number of implants per corpora lutea)
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured
before birth)
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 33,343,1987
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 3250 ug/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 20,87,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 3250 ug/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Maternal Effects - uterus, cervix, vagina
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 20,87,1980
丁酸氢化可的松 分子结构与计算化学数据
计算化学数据
1.疏水参数计算参考值(XlogP):无
2.氢键供体数量:2
3.氢键受体数量:6
4.可旋转化学键数量:6
5.互变异构体数量:15
6.拓扑分子极性表面积:101
7.重原子数量:31
8.表面电荷:0
9.复杂度:817
10.同位素原子数量:0
11.确定原子立构中心数量:7
12.不确定原子立构中心数量:0
13.确定化学键立构中心数量:0
14.不确定化学键立构中心数量:0
15.共价键单元数量:1
湖北诺纳科技有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:99%
包装信息:1kg铝箔袋/氟化瓶,10kg铝听/纸箱,25kg纸板桶/编织袋/塑料桶
价格:1.0元 - 25.0元
- 联系方式:
-
联系人:张经理
电话:027-59209883
手机:17386169806
湖北云镁科技有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:99%
包装信息:211kg,1kg铝箔袋/氟化瓶,10kg铝听/纸箱,25kg纸板桶/编织袋/塑料桶
价格:1.0元 - 211.0元
- 联系方式:
-
联系人:方经理
电话:027-59206672
手机:18327059871
山东默派生物科技有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:99%
包装信息:1kg
价格:洽谈
- 联系方式:
-
联系人:谭经理
电话:13053322091
手机:13053322091
上海源叶生物科技有限公司
- 产品介绍:
-
产品名称:13609-67-1
Cas号:13609-67-1
纯度:98%
包装信息:50mg
价格:140.0元
- 联系方式:
-
联系人:Yuki
电话:021-61845781
手机:13585604150
上海瀚思化工有限公司
- 产品介绍:
-
产品名称:17-丁酸氢化可的松酯
Cas号:13609-67-1
纯度:>98.0%(HPLC)
包装信息:1g
价格:697.0元
- 联系方式:
-
联系人:张
电话:021-64806089
手机:18049821080
杭州鼎燕化工有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:99%
包装信息:1kg
价格:100.0元
- 联系方式:
-
联系人:王芳
电话:0571-87157530
手机:13868066926
湖北恩星生物科技有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:
包装信息:100kg,200kg,300kg,400kg,500kg
价格:64.0元 - 100.0元
- 联系方式:
-
联系人:邵经理
电话:16621771607
手机:16621771607
武汉园星生物医药技术有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:
包装信息:100kg,200kg
价格:0.0元 - 100.0元
- 联系方式:
-
联系人:谢先生
电话:027-85558552
手机:15927172400
苏州市森菲达化工有限公司
- 产品介绍:
-
产品名称:丁酸氢化可的松
Cas号:13609-67-1
纯度:
包装信息:200kg
价格:洽谈
- 联系方式:
-
联系人:吴
电话:15195660023
手机:15195660023
