CClN

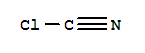
如图所示 制备氯化氰的装置
制法 氯与氰化钠作用制取
NaCN+Cl2=NaCl+CNCl
装置如图所示。500mL三颈瓶装有汞封搅拌,导气管和出气管。导入的气体在导入前要通过装硫酸的干燥瓶干燥。出气管与蛇形冷凝管相连,用冰盐混合物冷却,冷凝管末端一广口接受管,插入125mL锥形瓶中。接受瓶与装硫酸的洗气瓶相连。洗气瓶中有一足够粗的捕集管,防止硫酸倒吸。
把40g干燥的氰化钠粉末和140mL CCl4放在反应瓶中。将烧瓶及其内容物浸没在冰盐混合剂中直到温度降至-5~-10℃。用氮气排除仪器中的空气,在反应混合物中加3~4mL冰醋酸,开搅拌,通入氯气。反应瓶在全部时间应在-5℃或更低。氯气流速控制到不能有氯气从出气的洗瓶通过。反应经4~4.5h完成。将接受瓶放在以干冰冷却至-40~-50℃的丙酮中,并用氮气流代替氯气。这时使反应瓶外浴器温热在1~1.5h升温到60~65℃。此时在仪器中通入缓慢氮气流。当蒸馏完成后移开接受瓶,连接一根41mm长直径19mm的分馏柱。分馏柱四周包以用干冰冷却到-25℃的丙酮,对烧瓶微热,使粗氯化氰回流并逐出过量氯气。纯制完成后氯化氰为浅黄色。移开分馏柱,将此锥形瓶与一个接受瓶连接,接受瓶是容积35~40mL的泡球,其上熔接一根8mm的玻璃管。该玻璃管可以在收集产品后熔封。接受瓶用干冰冷却到-15℃~-20℃的丙酮冷却。产量36~39g,产率72%~78%。氯化氰有毒,制备在通风良好的橱中进行,操作者备有防毒面具。
用于有机合成。[18]
1.稳定性[12] 稳定
2.禁配物[13] 水、碱类、醇类、酸类
3.避免接触的条件[14] 受热、潮湿空气
4.聚合危害[15] 不聚合
5.分解产物[16] 氯化氢、氰化氢
1.性状:无色液体或气体,有催泪性。[1]
2.熔点(℃):-6.5~-6[2]
3.沸点(℃):12.5~13.1[3]
4.相对密度(水=1):1.186[4]
5.相对蒸气密度(空气=1):2.16[5]
6.饱和蒸气压(kPa):134.63(20℃)[6]
7.临界压力(MPa):5.99[7]
8.辛醇/水分配系数:-0.38[8]
9.溶解性:溶于水、乙醇、乙醚等多数有机溶剂。[9]
1.急性毒性[10]
LD50:6mg/kg(猫经口)
LC50:3124ppm(大鼠吸入,60min);4701ppm(大鼠吸入,30min)
2.刺激性 暂无资料
1.生态毒性 暂无资料
2.生物降解性 暂无资料
3.非生物降解性 暂无资料
4.其他有害作用[11] 该物质对环境有危害,应特别注意对水体的污染。
|
第一部分:化学品名称 |
1、摩尔折射率:11.44
2、摩尔体积(cm3/mol):49.8
3、等张比容(90.2K):118.6
4、表面张力(dyne/cm):32.2
5、极化率(10-24cm3):4.53
1.疏水参数计算参考值(XlogP):1
2.氢键供体数量:0
3.氢键受体数量:1
4.可旋转化学键数量:0
5.互变异构体数量:无
6.拓扑分子极性表面积23.8
7.重原子数量:3
8.表面电荷:0
9.复杂度:31.3
10.同位素原子数量:0
11.确定原子立构中心数量:0
12.不确定原子立构中心数量:0
13.确定化学键立构中心数量:0
14.不确定化学键立构中心数量:0
15.共价键单元数量:1