C12H4Cl4O2
 F;
F; Xn
Xn
| 国标编号: | |
| CAS: | 1746-01-6 |
| 中文名称: | 二恶英 |
| 英文名称: | Dibenzo-p-Dioxin;Dioxin |
| 别 名: | TCDD |
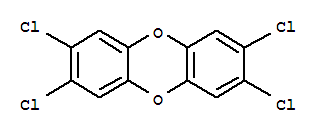
| 分子式: | C12H4Cl4O2 |
| 分子量: | 321.96 |
| 熔 点: | 302~305℃ |
| 密 度: | |
| 蒸汽压: | |
| 溶解性: | |
| 稳定性: | |
| 外观与性状: | |
| 危险标记: | 白色结晶体 |
| 用 途: | 在制造氯酚的过程中会产生二恶英 |
一、健康危害
动物试验: 对胎儿有毒性,胎儿发育异常,胎儿死亡。 对胎儿和胚胎有影响,对胎儿血液和淋巴系统有影响,对新生儿生长有影响。 对胎儿泌尿、生殖系统有影响,对成活分娩指数(可存活数/出生总数),断奶和授乳指数(断奶尚存活数/第四天存活数)有影响。 按RTECS标准为致癌物,肝及甲状腺肿瘤,皮肤肿瘤。
二、毒理学资料及环境行为
急性毒性:LD5022500ng/kg(大鼠经口);114μg/kg(小鼠经口);500μg/kg(豚鼠经口) 刺激性:兔经眼:2mg,中等刺激 致突变:微生物突变-鼠伤寒沙门氏菌,3mg/L ; 微生物突变-大肠杆菌,2mg/L 致癌性判定:动物和人皆为不肯定性反应。 二恶英在500℃开始分解,800℃时,21秒内完全分解。二恶英在土壤内残留时间为10年。
来源:在制造氯酚的过程中会产生二恶英,生成的量取决于过程的压力和温度。生产除草剂2,4,5-三氯苯酚时,1,2,4,5-四氯苯碱解时,会产生二恶英。农药2,4,5-涕中含有二恶英杂质。
3.现场应急监测方法
4.实验室监测方法
色谱/质谱法《固体废弃物试验分析评价手册》中国环境监测总站等译 测量大型堆料场净化工段附近环境空气中2,3,7,8-四氯二苯并-P-二恶英用的方法[刊,英]/Fairless B.J.;Bates D.I.,Hudson J.…//Environ.Sci.Technol.-1987,21(6).-550~555
5.环境标准| 中国(98年修改稿) | 地表水Ⅰ、Ⅱ、Ⅲ类水域有机化学物质特定项目标准值 | 3.0×10-8mg/L (2,3,7,8-TCDD) |
| 中国(GWKB3-2000) | 生活垃圾焚烧污染控制标准 | 焚烧炉大气污染物排放限值 1.0ng/m3(测定均值) |
1.疏水参数计算参考值(XlogP):无
2.氢键供体数量:0
3.氢键受体数量:2
4.可旋转化学键数量:0
5.互变异构体数量:无
6.拓扑分子极性表面积:18.5
7.重原子数量:18
8.表面电荷:0
9.复杂度:259
10.同位素原子数量:0
11.确定原子立构中心数量:0
12.不确定原子立构中心数量:0
13.确定化学键立构中心数量:0
14.不确定化学键立构中心数量:0
15.共价键单元数量:1